Thermodynamic Quantities for Substances and Ions at 25oC.
Inorganic Substances
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) | So (J/mol•K) | |
| e–(g) | 0 | 0 | 20.87 |
Aluminum
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Al(s) | 0 | 0 | 28.3 |
| Al3+(aq) | –531 | –485 | –321.7 |
| AlCl3(s) | –705.6 | –630.1 | 109.3 |
| Al2Cl6(g) | –1291 | –1221 | 490 |
| AlF3(s) | –1504 | –1425 | 66.48 |
Al2O3(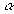 , s ) , s ) | –1676 | –1582 | 50.94 |
| Al(OH)3(s) | –1276 | – | – |
| Al2(SO4)3(s) | –3441 | –3100 | 239 |
Barium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Ba(s) | 0 | 0 | 62.3 |
| Ba2+(aq) | –537.6 | –560.8 | 9.6 |
| Ba2+(g) | 1649.9 | ||
| Ba(g) | 175.6 | 144.8 | 170.28 |
| Ba+(g) | 684.6 | ||
| BaCO3(s) | –1216 | –1138 | 112 |
| BaCl2(s) | –858.1 | –810.4 | 123.7 |
| BaF2(s) | –1209 | –1159 | 96.40 |
| BaO(s) | –548.1 | –520.4 | 72.09 |
| Ba(OH)2(s) | –946.0 | –859.4 | 107 |
| Ba(OH)2•8H2O(s) | –3342 | –2793 | 427 |
| BaSO4(s) | –1473 | –1362 | 132 |
Beryllium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Be(s) | 0 | 0 | 9.54 |
| BeCl2(s) | –496.2 | –449.5 | 75.81 |
| BeF2(s) | –1027 | –979.5 | 53.35 |
| BeO(s) | –608.4 | –579.1 | 13.77 |
Bismuth
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Bi(s) | 0 | 0 | 56.74 |
| BiCl3(s) | –379 | –315 | 177 |
| Bi2O3(s) | –573.9 | –493.7 | 151 |
Boron
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
B(s,  -rhomohedral) -rhomohedral) | 0 | 0 | 5.86 |
| BCl3(l) | –427.2 | –387 | 206 |
| BF3(g) | –1137 | –1120.3 | 254.0 |
| B2H6(g) | 36 | 86.6 | 232.0 |
| B2O3(s) | –1272 | –1193 | 53.8 |
Bromine
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Br(g) | 111.9 | 82.40 | 174.90 |
| Br–(aq) | –120.9 | –102.82 | 80.71 |
| Br–(g) | –218.9 | ||
| Br2(g) | 30.91 | 3.13 | 245.38 |
| Br2(l) | 0 | 0 | 152.23 |
| BrCl(g) | 14.6 | –0.96 | 240.0 |
| BrF3(g) | –255.6 | –229.5 | 292.4 |
| BrF3(l) | –300.8 | –240.6 | 178.2 |
| HBr(g) | –36.40 | –53.43 | 198.6 |
Cadmium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Cd(s) | 0 | 0 | 51.76 |
| Cd(g) | 112.8 | 78.20 | 167.64 |
| Cd2+(aq) | –75.90 | –77.61 | –73.2 |
| CdCl2(s) | –391.5 | –344.0 | 115.3 |
| CdO(s) | –258 | –228 | 54.8 |
| CdS(s) | –144 | –141 | 71 |
Calcium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Ca(s) | 0 | 0 | 41.4 |
| Ca(g) | 192.6 | 158.9 | 154.78 |
| Ca2+(aq) | –542.96 | –553.04 | –55.2 |
| Ca2+(g) | 1934.1 | ||
| Ca+(g) | 788.6 | ||
| CaBr2(s) | –682.8 | –663.6 | 130. |
| CaCO3(s) | –1207 | –1128 | 88.70 |
| CaCl2(s) | –795.8 | –748.1 | 105 |
| CaF2(s) | –1220 | –1167 | 68.87 |
| CaH2(s) | –186 | –147 | 42 |
| Ca(NO3)2(s) | –938.4 | –743.2 | 193 |
| CaO(s) | –635.1 | –604.0 | 39.75 |
| Ca(OH)2(s) | –986.1 | –898.6 | 83.39 |
| CaOH+(aq) | –718.4 | ||
| Ca3(PO4)2(s) | –4121 | –3885 | 236 |
| CaSO4(s) | –1434 | –1322 | 106.7 |
Carbon
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| C(g) | 716.7 | 671.3 | 158.0 |
| C(s, graphite) | 0 | 0 | 5.74 |
| C(s, diamond) | 1.90 | 2.90 | 2.38 |
| CCl4(g) | –102.9 | –60.63 | 309.7 |
| CCl4(l) | –135.4 | –65.27 | 216.2 |
| C2N2(g) | 308.9 | 297.2 | 242.3 |
| CN–(aq) | 151 | 166 | 118 |
| HCN(g) | 135 | 125 | 201.7 |
| HCN(l) | 105 | 121 | 112.8 |
| HCN(aq) | 105 | 112 | 129 |
| CO(g) | –110.5 | –137.2 | 197.6 |
| CO2(g) | –393.5 | –394.4 | 213.6 |
| CO2(aq) | –412.9 | –386.2 | 121 |
| CO32–(aq) | –677.1 | –527.8 | –56.9 |
| HCO3–(aq) | –691.11 | –587.06 | 95.0 |
| H2CO3(aq) | –698.7 | –623.42 | 191 |
| C3O2(g) | –93.72 | –109.8 | 276.4 |
| C3O2(l) | –117.3 | –105.0 | 181.1 |
| COCl2(g) | –220.9 | –206.8 | 283.8 |
| CH3Cl(g) | –83.7 | –60.2 | 234 |
| CH2Cl2(l) | –117 | –63.2 | 179 |
| CHCl3(l) | –132 | –71.5 | 203 |
| COS(g) | –138.4 | –165.6 | 231.5 |
| CS2(g) | 117 | 66.9 | 237.79 |
| CS2(l) | 89.70 | 65.27 | 151.3 |
Cesium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Cs+(g) | 458.5 | 427.1 | 169.72 |
| Cs+(aq) | –248 | –282.0 | 133 |
| Cs(g) | 76.7 | 49.7 | 175.5 |
| Cs(s) | 0 | 0 | 85.15 |
| CsF(s) | –554.7 | –525.4 | 88 |
| CsCl(s) | –442.8 | –414 | 101.18 |
| CsBr(s) | –395 | –383 | 121 |
| CsI(s) | –337 | –333 | 130 |
Chlorine
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Cl(g) | 121.7 | 105.7 | 165.1 |
| Cl–(aq) | –167.2 | –131.2 | 56.5 |
| Cl–(g) | –234 | –240 | 153.25 |
| Cl2(g) | 0 | 0 | 223.0 |
| ClF3(g) | –163.2 | –123.0 | 281.5 |
| ClO2(g) | 102.5 | 120.5 | 256.7 |
| Cl2O(g) | 80.33 | 97.49 | 267.9 |
| HCl(g) | –92.31 | –95.30 | 186.8 |
| HCl(aq) | –167.2 | –131.3 | 56.48 |
Chromium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Cr(s) | 0 | 0 | 23.66 |
| CrCl2(s) | –395.4 | –356.0 | 115.3 |
| CrCl3(s) | –556.5 | –486.1 | 123.0 |
| Cr2O3(s) | –1135 | –1053 | 81.17 |
| CrO42–(aq) | –881.2 | –727.8 | 50.21 |
| Cr2O72–(aq ) | –1490.3 | –1301.1 | 261.9 |
| [Cr(H2O)6]3+(aq) | –1971 |
Cobalt
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Co(s) | 0 | 0 | 30.0 |
| Co2+(aq) | –67.4 | –51.5 | –155 |
| Co3+(aq) | 92.0 | 134.0 | –305.0 |
| CoO(s) | –237.9 | –214.2 | 52.97 |
| Co3O4(s) | –891.0 | –774.0 | 102.5 |
| Co(OH)2(s, pink) | –539.7 | –454.4 | 79.0 |
| CoCl2(s) | –312.5 | –269.8 | 109.2 |
Copper
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Cu(s) | 0 | 0 | 33.15 |
| Cu(g) | 341.1 | 301.4 | 166.29 |
| Cu+(aq) | 51.9 | 50.2 | –26 |
| Cu2+(aq) | 64.77 | 65.49 | –99.6 |
| CuCl2(s) | –220.1 | –175.7 | 108.1 |
| CuCO3•Cu(OH)2(s) | –1051 | –893.7 | 186 |
| Cu2O(s) | –168.6 | –146.0 | 93.14 |
| CuO(s) | –157.3 | –129.7 | 42.63 |
| Cu(OH)2(s) | –450.2 | –373 | 108 |
| CuSO4•5H2O(s) | –2279.6 | –1880.1 | 300.4 |
Fluorine
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| F(g) | 78.99 | 61.92 | 158.7 |
| F –(g) | –255.6 | –262.5 | 145.47 |
| F –(aq) | –332.6 | –278.8 | –13.8 |
| F2(g) | 0 | 0 | 202.7 |
| HF(g) | –271.1 | –273.2 | 173.7 |
Helium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| He(g) | 0 | 0 | 126.0 |
Hydrogen
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| H(g) | 218.0 | 203.3 | 114.6 |
| H+(g) | 1536.3 | 1517.1 | 108.83 |
| H+(aq) | 0 | 0 | 0 |
| H3O+(aq) | –285.8 | 69.91 | |
| H2(g) | 0 | 0 | 130.6 |
| HBr(g) | –36.40 | –53.43 | 198.6 |
| HCl(g) | –92.31 | –95.30 | 186.8 |
| HCl(aq) | –167.2 | –131.3 | 56.48 |
| HCN(g) | 135 | 125 | 201.7 |
| HF(g) | –271.1 | –273.2 | 173.7 |
| HI(g) | 26.48 | 1.72 | 206.5 |
| HNO3(l) | –173.2 | –79.91 | 155.6 |
| HNO3(aq) | –207.4 | –113.3 | 146.4 |
| H2O(g) | –241.8 | –228.6 | 188.7 |
| H2O(l) | –285.8 | –237.2 | 69.91 |
| H2O2(g) | –136.1 | –105.5 | 232.9 |
| H2O2(l) | –187.8 | –120.4 | 110 |
| H2O2(aq) | –191.2 | –134.1 | 144 |
| H2S(g) | –20.63 | –33.56 | 205.7 |
| H2SO4(l) | –814.0 | –690.1 | 156.9 |
| H2SO4(aq) | –909.3 | –744.6 | 20.08 |
Iodine
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| I(g) | 106.8 | 70.28 | 180.7 |
| I–(g) | –194.7 | ||
| I–(aq) | –55.19 | –51.57 | 111.3 |
| I2(g) | 62.44 | 19.36 | 260.6 |
| I2(s) | 0 | 0 | 116.1 |
| IBr(g) | 40.84 | 3.72 | 258.7 |
| ICl(g) | 17.78 | –5.44 | 247.4 |
| ICl(l) | –23.89 | –13.60 | 135.1 |
| HI(g) | 26.48 | 1.72 | 206.5 |
Iron
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Fe(s) | 0 | 0 | 27.28 |
| Fe(l) | 13.13 | 11.05 | 34.29 |
| Fe2+(aq) | –89.1 | –78.90 | –137.7 |
| Fe3+(aq) | –48.5 | –4.7 | –315.9 |
| FeCO3(s) | –740.6 | –666.7 | 92.88 |
| FeCl2(s) | –341.8 | –302.3 | 118.0 |
| FeCl3(s) | –399.5 | –334.1 | 142.3 |
| FeO(s) | –272.0 | –251.5 | 60.75 |
| Fe2O3(s) | –824.2 | –742.2 | 87.40 |
| Fe3O4(s) | –1118.4 | –1015.4 | 146.4 |
| Fe(OH)3(s) | –823.0 | –696.6 | 107 |
Lead
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Pb(s) | 0 | 0 | 64.81 |
| Pb2+(aq) | –1.7 | –24.43 | 10.5 |
| PbBr2(s) | –278.7 | 161.5 | |
| PbCl2(s) | –359 | –314 | 136 |
| PbI2(s) | –175.5 | –173.6 | 174.8 |
| PbO(s, red) | –219.0 | –189.2 | 66.5 |
| PbO2(s) | –277 | –217.4 | 68.6 |
| PbS(s) | –98.3 | –96.7 | 91.3 |
| PbSO4(s) | –919.9 | –813.2 | 148.6 |
Lithium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Li(s) | 0 | 0 | 29.12 |
| Li+(aq) | –278.5 | –293.3 | 13.4 |
| Li(g) | 161 | 128 | 138.67 |
| Li+(g) | 687.163 | 649.989 | 132.91 |
| LiF(s) | –616.9 | –588.7 | 35.66 |
| LiCl(s) | –408.6 | –384.4 | 59.33 |
| LiBr(s) | –351 | –342 | 74.1 |
| LiI(s) | –270 | –270 | 85.8 |
| Li2O(s) | –597.94 | –561.18 | 37.57 |
| LiOH(s) | –484.9 | –439.0 | 42.80 |
| LiNO3(s) | –483.1 | –381.1 | 90.0 |
Magnesium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Mg(s) | 0 | 0 | 32.69 |
| Mg(g) | 150 | 115 | 148.55 |
| Mg+(g) | 894.1 | ||
| Mg2+(g) | 2351 | ||
| Mg2+(aq) | –466.9 | –454.8 | –138.1 |
| MgCl2(s) | –641.3 | –591.8 | 89.62 |
| MgCO3(s) | –1096 | –1012 | 65.7 |
| MgF2(s) | –1124 | –1071 | 57.24 |
| MgO(s) | –601.7 | –569.4 | 26.94 |
| MgOH+(aq) | –626.7 | ||
| Mg(OH)2(s) | –924.7 | –833.9 | 63.18 |
| Mg3N2(s) | –461 | –401 | 88 |
| MgSO4(s) | –1285 | –1171 | 91.6 |
Manganese
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Mn(s, ) | 0 | 0 | 32.0 |
| Mn2+(aq) | –220.8 | –228.1 | –73.6 |
| MnCl2(s) | –481.3 | –440.5 | 118.2 |
| MnO2(s) | –520 | –465.2 | 53.05 |
| MnO4–(aq) | –541.4 | –447.2 | 191.2 |
| MnO42–(aq) | –653.0 | –500.7 | 59.0 |
Mercury
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Hg(g) | 61.32 | 31.85 | 174.9 |
| Hg(l) | 0 | 0 | 76.02 |
| Hg2+(aq) | 164.8 | ||
| Hg22+(aq) | 172.4 | 153.9 | 84.5 |
| HgCl2(s) | –230 | –184 | 144 |
| Hg2Br2(s) | –206.9 | 218.0 | |
| Hg2Cl2(s) | –265.4 | –210.66 | 191.6 |
| Hg2I2(s) | –121.3 | 233.5 | |
| HgO(s) | –90.83 | –58.56 | 70.29 |
Nickel
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Ni(s) | 0 | 0 | 30.1 |
| Ni2+(aq) | –64.0 | –46.4 | –159 |
| NiCl2(s) | –305.3 | –259.0 | 97.7 |
| Ni(OH)2(s) | –529.7 | –447.2 | 88.0 |
Nitrogen
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| N(g) | 472.7 | 455.6 | 153.2 |
| N2(g) | 0 | 0 | 191.5 |
| NF3(g) | –124.7 | –83.2 | 260.7 |
| NH3(g) | –46.11 | –16.48 | 192.3 |
| NH3(aq) | –80.29 | –26.57 | 111.3 |
| NH4+(aq) | –132.5 | –79.31 | 113.4 |
| NH4Br(s) | –270.8 | –175 | 113.0 |
| NH4Cl(s) | –314.4 | –203.0 | 94.56 |
| NH4F(s) | –464.0 | –348.8 | 71.96 |
| NH4HCO3(s) | –849.4 | –666.1 | 121 |
| NH4I(s) | –201.4 | –113 | 117 |
| NH4NO3(s) | –365.6 | –184.0 | 151.1 |
| NH4NO3(aq) | –339.9 | –190.7 | 259.8 |
| (NH4)2SO4(s) | –1181 | –901.9 | 220.1 |
| N2H4(g) | 95.40 | 159.3 | 238.4 |
| N2H4(l) | 50.63 | 149.2 | 121.2 |
| NO(g) | 90.25 | 86.57 | 210.6 |
| N2O(g) | 82.05 | 104.2 | 219.7 |
| NO2(g) | 33.18 | 51.30 | 240.0 |
| N2O4(g) | 9.16 | 97.82 | 304.2 |
| N2O4(l) | –19.6 | 97.40 | 209.2 |
| N2O5(g) | 11.3 | 115.1 | 355.7 |
| NO3–(aq) | –205.0 | –108.7 | 146.4 |
| HNO3(l) | –173.2 | –79.91 | 155.6 |
| HNO3(aq) | –207.4 | –113.3 | 146.4 |
| NOBr(g) | 82.17 | 82.4 | 273.5 |
| NOCl(g) | 51.71 | 66.07 | 261.6 |
Oxygen
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| O(g) | 249.2 | 231.7 | 160.9 |
| O2(g) | 0 | 0 | 205.0 |
| O3(g) | 142.7 | 163.2 | 238.8 |
| OH–(aq) | –230.0 | –157.2 | –10.75 |
| OF2(g) | 24.5 | 41.8 | 247.3 |
Phosphorous
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| P(g) | 333.9 | 292.0 | 163.1 |
| P( | 0 | 0 | 41.1 |
| P4(s, red) | –17.6 | –12.1 | 22.8 |
| P2(g) | 146.2 | 103.8 | 218.0 |
| P4(g) | 58.9 | 24.5 | 279.9 |
| PCl3(g) | –287.0 | –267.8 | 311.7 |
| PCl3(l) | –319.7 | –272.3 | 217.1 |
| PCl5(g) | –374.5 | –305.0 | 364.5 |
| PCl5(l) | –443.5 | – | – |
| PH3(g) | 5.4 | 13.4 | 210.1 |
| P4O10(s) | –2984 | –2698 | 228.9 |
| PO43–(aq) | –1277 | –1019 | –222 |
| HPO42–(aq) | –1292.1 | –1089.3 | –33 |
| H2PO4–(aq) | –1296.3 | –1130.4 | 90.4 |
| H3PO4(aq) | –1288.3 | –1142.7 | 158 |
Potassium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| K(g) | 89.24 | 60.63 | 160.2 |
| K(l) | 2.28 | 0.26 | 71.46 |
| K(s) | 0 | 0 | 64.18 |
| K+(aq) | –252.4 | –283.3 | 102.5 |
| K+(g) | 514.197 | 481.202 | 154.47 |
| KBr(s) | –393.8 | –380.7 | 95.90 |
| KCN(s) | –113 | –101.9 | 128.5 |
| KCl(s) | –436.7 | –409.2 | 82.59 |
| KClO3(s) | –397.7 | –296.3 | 143 |
| KClO4(s) | –432.8 | –303.2 | 151.0 |
| KF(s) | –567.3 | –537.8 | 66.57 |
| KI(s) | –327.9 | –324.9 | 106.3 |
| KNO3(s) | –494.6 | –394.9 | 133.1 |
| KOH(s) | –424.8 | –379.1 | 78.87 |
| KOH(aq) | –482.4 | –440.5 | 91.63 |
| K2SO4(s) | –1438 | –1321 | 175.6 |
Rubidium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Rb+(g) | 495.04 | ||
| Rb+(aq) | –246 | –282.2 | 124 |
| Rb(g) | 85.81 | 55.86 | 169.99 |
| Rb(s) | 0 | 0 | 69.5 |
| RbF(s) | –549.28 | ||
| RbCl(s) | –430.58 | ||
| RbBr(s) | –389.2 | –378.1 | 108.3 |
| RbI(s) | –328 | –326 | 118.0 |
Silicon
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Si(s) | 0 | 0 | 18.8 |
| SiH4(g) | 34 | 56.9 | 204.5 |
| Si2H6(g) | 80.3 | 127 | 272.5 |
| SiO2(s, quartz) | –910.9 | –856.5 | 41.84 |
Silver
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Ag(s) | 0 | 0 | 42.55 |
| Ag(g) | 289.2 | 250.4 | 172.892 |
| Ag+(aq) | 105.6 | 77.11 | 72.68 |
| AgBr(s) | –100.4 | –96.90 | 107 |
| AgCl(s) | –127.1 | –109.8 | 96.2 |
| AgF(s) | –203 | –185 | 84 |
| AgI(s) | –61.84 | –66.19 | 115 |
| AgNO3(s) | –124.4 | –33.5 | 140.9 |
| Ag2O(s) | –31.0 | –11.2 | 121 |
| Ag2S(s) | –31.8 | –40.3 | 146 |
| Ag2SO4(s) | –715.9 | –618.5 | 200.4 |
Sodium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Na(g) | 107.3 | 76.78 | 153.6 |
| Na(l) | 2.41 | 0.50 | 57.86 |
| Na(s) | 0 | 0 | 51.21 |
| Na+(aq) | –240.1 | –261.9 | 59.0 |
| Na+(g) | 609.839 | 574.877 | 147.85 |
| Na2(g) | 142.0 | 104.0 | 230.1 |
| NaBr(s) | –361.1 | –349.0 | 86.82 |
| Na2CO3(s) | –1131 | –1044 | 135.0 |
| NaHCO3(s) | –950.8 | –851.0 | 102 |
| NaCl(s) | –411.1 | –384.0 | 72.13 |
| NaCl(aq) | –407.3 | –393.1 | 115.5 |
| NaClO3(s) | –365.8 | –262.3 | 123 |
| NaClO4(s) | –383.3 | –254.9 | 142.3 |
| NaF(s) | –573.7 | –543.5 | 51.46 |
| NaH(s) | –56.27 | –33.5 | 40.02 |
| NaI(s) | –287.8 | –286.1 | 98.53 |
| NaNO3(s) | –467.9 | –367.1 | 116.5 |
| NaNO3(aq) | –447.4 | –373.2 | 205.4 |
| Na2O2(s) | –510.9 | –447.7 | 94.98 |
| NaOH(s) | –425.6 | –379.5 | 64.48 |
| NaOH(aq) | –469.2 | –419.2 | 48.1 |
| NaH2PO4(s) | –1537 | –1386 | 127.5 |
| Na2HPO4(s) | –1748 | –1608 | 150.5 |
| Na3PO4(s) | –1917 | –1789 | 173.8 |
| NaHSO4(s) | –1125 | –992.9 | 113 |
| Na2SO4(s) | –1387 | –1270 | 149.6 |
| Na2SO4(aq) | –1390 | –1268 | 138.1 |
| Na2SO4•10H2O(s) | –4327 | –3647 | 592.0 |
| Na2S2O3(s) | –1123 | –1028 | 155 |
Strontium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Sr2+(g) | 1784 | ||
| Sr2+(aq) | –545.8 | –557.3 | –32.6 |
| Sr+(g) | 719.6 | ||
| Sr(g) | 164 | 110 | 164.54 |
| Sr(s) | 0 | 0 | 54.4 |
| SrCl2(s) | –828.4 | –781.2 | 117 |
| SrF2(s) | –1216.3 | 82.1 | |
| SrO(s) | –592.0 | –562.4 | 55.5 |
| SrCO3(s) | –1218 | –1138 | 97.1 |
| SrSO4(s) | –1445 | –1334 | 122 |
Sulfur
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| S(s, rhombic) | 0 | 0 | 31.8 |
| S(s, monoclinic) | 0.30 | 0.096 | 32.6 |
| S8(g) | 102.3 | 49.16 | 430.2 |
| S(g) | 279 | 239 | 168 |
| S2(g) | 129 | 80.1 | 228.1 |
| S2–(aq) | 41.8 | 83.7 | 22 |
| HS–(aq) | –17.7 | 12.6 | 61.1 |
| H2S(g) | –20.63 | –33.56 | 205.7 |
| H2S(aq) | –39 | –27.4 | 122 |
| S2Cl2(g) | –18.4 | –31.8 | 331.5 |
| SF6(g) | –1209 | –1105 | 291.7 |
| SO2(g) | –296.8 | –300.2 | 248.1 |
| SO3(g) | –395.7 | –371.1 | 256.6 |
| SO42–(aq) | –909.3 | –744.5 | 20.1 |
| HSO4–(aq) | –885.75 | –752.87 | 126.9 |
| H2SO4(l) | –814.0 | –690.1 | 156.9 |
| H2SO4(aq) | –909.3 | –744.6 | 20.08 |
| S2O32–(aq ) | –648.5 | –522.5 | 67 |
| SO2Cl2(g) | –364.0 | –320.0 | 311.8 |
| SO2Cl2(aq) | –394.1 | –314 | 207 |
Tin
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Sn(s, white) | 0 | 0 | 51.55 |
| Sn(s, gray) | –2.1 | 0.1 | 44.14 |
| SnCl4(l) | –511.3 | –440.2 | 259 |
| SnO(s) | –286 | –257 | 56.5 |
| SnO2(s) | –580.7 | –519.7 | 52.3 |
Titanium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Ti(s) | 0 | 0 | 30.6 |
| TiCl2(s) | –513.8 | –464.4 | 87.4 |
| TiCl3(s) | –720.9 | –653.5 | 139.7 |
| TiCl4(g) | –763.2 | –726.8 | 355 |
| TiCl4(l) | –804.2 | –737.2 | 252.3 |
| TiO2(s) | –944.7 | –889.5 | 50.33 |
Uranium
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| U(s) | 0 | 0 | 50.21 |
| UF6(g) | –2147 | –2064 | 378 |
| UF6(s) | –2197 | –2069 | 228 |
| UO2(s) | –1085 | –1032 | 77.03 |
Zinc
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| Zn(s) | 0 | 0 | 41.6 |
| Zn(g) | 130.5 | 94.93 | 160.9 |
| Zn2+(aq) | –153.9 | –147.1 | –112.1 |
| ZnCl2(s) | –415.1 | –369.4 | 111.5 |
| Zn(OH)2(s) | –641.9 | –553.5 | 81.2 |
| ZnO(s) | –348.3 | –318.3 | 43.64 |
| ZnS(s, zincblende) | –203 | –198 | 57.7 |
Organic Substances
| Substance or Ion |  Hfo (kJ/mol) Hfo (kJ/mol) |  Gfo (kJ/mol) Gfo (kJ/mol) | So (J/mol•K) |
| CH4(g) methane | –74.81 | –50.75 | 186.2 |
| C2H2(g) acetylene | 226.7 | 209.2 | 200.8 |
| C2H4(g) ethylene | 52.26 | 68.12 | 219.4 |
| C2H6(g) ethane | –84.68 | –32.89 | 229.5 |
| C3H8(g) propane | –103.8 | –23.56 | 270.2 |
| C4H10(g) butane | –125.7 | –17.15 | 310.1 |
| C6H6(g) benzene | 82.93 | 129.7 | 269.2 |
| C6H6(l) benzene | 48.99 | 124.4 | 173.3 |
| C6H12(g) cyclohexane | –123.1 | 31.8 | 298.2 |
| C6H12(l) cyclohexane | –156.2 | 26.7 | 204.3 |
| C10H8(g) naphthalene | 149 | 223.6 | 335.6 |
| C10H8(s) naphthalene | 75.3 | 201.0 | 166.9 |
| HCHO(g) formaldehyde | –117.0 | –110.0 | 218.7 |
| CH3OH(g) methanol | –200.7 | –162.0 | 239.7 |
| CH3OH(l) methanol | –238.7 | –166.4 | 126.8 |
| CH3CHO(g) acetaldehyde | –166.1 | –133.4 | 246.4 |
| CH3CHO(l) acetaldehyde | –191.8 | –128.3 | 160.4 |
| C2H5OH(g) ethanol | –234.4 | –167.9 | 282.6 |
| C2H5OH(l) ethanol | –277.7 | –174.9 | 160.7 |
| C6H5OH(s) phenol | –165.0 | –50.42 | 144.0 |
| (CH3)2CO(g) acetone | –216.6 | –153.1 | 294.9 |
| (CH3)2CO(l) acetone | –247.6 | –155.7 | 200.4 |
| HCOOH(l) formic acid | –409 | –346 | 129.0 |
| HCOOH(aq) formic acid | –410 | –356 | 164 |
| HCOO–(aq) formate ion | –410 | –335 | 91.6 |
| CH3COOH(g) acetic acid | –432.3 | –374.0 | 282.5 |
| CH3COOH(l) acetic acid | –484.1 | –389.9 | 159.8 |
| CH3COOH(aq) acetic acid | –488.3 | –396.6 | 178.7 |
| C6H5COOH(s) benzoic acid | –385.1 | –245.3 | 167.6 |
| CH3NH2(g) methylamine | –23.0 | 32.3 | 242.6 |
| C6H5NH2(g) aniline | 86.86 | 166.7 | 319.2 |
| C6H5NH2(l) aniline | 31.6 | 149.1 | 191.3 |
| C6H12O6(s) glucose | –1273.3 | –910.4 | 212.1 |
| C12H22O11(s) sucrose | –2226.1 | 360.2 |
fonte
mmmm